LILRB family cell screening model
In the tumor microenvironment (TME), complex interactions occur between various cell types, including tumor cells, immune cells, fibroblasts, mesenchymal stem cells, endothelial cells, pericytes, adipocytes, and neurons cells etc. Among them, the molecular mechanism represented by PD-L1/PD-1 interaction is an important discovery of the interaction between tumor cells and T cells. The understanding of its molecular mechanism has led to the development of immune checkpoint biology and tumor immunotherapy. A revolutionary breakthrough. Nonetheless, PD-1-based immune checkpoint blockade therapy is effective in only a small proportion of cancer patients, suggesting that other mechanisms of immune evasion exist, and T cells constitute only a small fraction of immune cells in the TME. The most abundant type of immune cells in the TME are myeloid cells, and in cancer patients, systemic and local signals convert many myeloid cells to immunosuppressive cells, including myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs) and resistant dendritic cells. These immunosuppressive myeloid cells suppress the antitumor immune activity of cells including T cells and natural killer (NK), and support tumor cell growth, angiogenesis, and metastasis. A better understanding of the interactions between myeloid cells, T cells, and tumor cells in the TME will facilitate the development of new drugs and approaches for tumor immunotherapy.
Leukocyte Ig-like receptor B subfamily (LILRB) proteins and the related receptor LAIR1 are type I immunosuppressive receptors that are expressed specifically in primates and are usually expressed only on hematopoietic cells, which have ligand-binding cells An outer Ig-like domain that recruits the intracellular immunoreceptor tyrosine inhibitory motif (ITIM) of the phosphatases SHP1, SHP2, or SHIP. Recent studies have shown that these receptors may have the function of linking multiple cell types in the TME and provide new opportunities for anticancer immunotherapy.
Although different LILRBs as well as LAIR1 share the same domain, each specific LILRB and LAIR1 can be expressed by different cell types and exert correspondingly different functions in tumor immunology. Although, compared with the broad expression of LILRB1, it can inhibit the activity of NK cells and T cells and the phagocytosis of macrophages, but LILRB1 is not necessarily the main inhibitory receptor in these cells, and LILRB1 blockade is associated with other anticancer drugs. The combination of treatments, however, appears to synergistically boost immune cell function. LILRB2 is predominantly expressed by myeloid cells and serves as a major inhibitory receptor for MDSCs and TAMs; LILRB3 is also expressed by myeloid cells and contributes to the immunosuppressive activity of the TME; there is evidence that monocyte-expressed LILRB4 supports Antigen presentation and other activities of immunosuppressive myeloid cells, while LILRB4 also appears to be an attractive target for the treatment of monocytic leukemia and certain B-cell malignancies, mainly due to its specific expression on these types of tumors . The least studied member of the LILRB family is LILRB5, whose role in tumor immunology is unclear. In contrast, LAIR1 is expressed on most cells of the hematopoietic lineage and suppresses the activity of several immune cell types in the TME, mediating signaling to regulate the development of certain myeloid and lymphocytic leukemias.
LILRBs and LAIR1 are generally activated by a variety of ligands (as shown in the table below), and the ligands of LILRBs and LAIR1 can be expressed by different types of cells in the TME and may lead to different interactions and functions between these cells. For example, LILRB2 on myeloid cells may be activated by MHC-I expressed by cancer cells, SEMA4A expressed by antigen-presenting cells, CD1d expressed by monocytes, myelin inhibitor expressed by neurons, and β-amyloid. Additionally, in some types of cancer, soluble ligands produced by LILRB2-expressing myeloid cells, such as Angptl2, initiate autocrine signaling through LILRB2; these soluble ligands may support LILRB2 that maintains the activity of immunosuppressive myeloid cells in the TME Basic activation of . LILRB4 expressed on TME monocytes may be activated by ApoE and fibronectin expressed by macrophages through trans- or cis-interactions. These complex ligand/receptor interactions suggest that immune function mediated by LILRB/LAIR1 family members is multidimensional.
Currently, various forms of drugs or treatment options based on LILRBs and LAIR1-mediated signaling have been developed, including monoclonal antibodies and derivatives, recombinant proteins, and cell therapy (as shown in the table below). Multiple antagonist antibodies against LILRB1, LILRB2, LILRB4, and LAIR1 are already in clinical trials for the treatment of various tumors; in addition, recombinant extracellular domains of certain inhibitory receptors may also have therapeutic potential, an example is based on LAIR2 The recombinant protein of LAIR1 can compete with LAIR1 for collagen binding, thereby releasing its immunosuppressive effect. Whether targeting the TME of solid cancers or hematological malignancies, the antagonistic properties of anti-LILRB or anti-LAIR1 antibodies have been proven to be feasible, as these antibodies have been shown to activate anti-tumor activity by blocking receptor-ligand interactions Considering the interaction between tumor cells, T cells and myeloid cells, combination therapy targeting multiple cell types above, such as the combination of anti-LILRB and T cell checkpoint inhibitors, may be a way to gain Immunotherapy strategies for long-term antitumor efficacy.

For members of the LILRBs family, we have also developed a series of cell screening models, which can be used for functional screening and activity testing of related antagonist antibody drugs.
LILRB4 Effector Reporter Cell RQP74102
ANGPTLx/TCR activitor/CHO RQP74103
APOE/TCR Activator/CHO RQP74107

Figure 1. Dose response of LILRB4 Blocking antibody in LILRB4 Effector Reporter Cells(C5) With ANGPTLx TCR Activitor CHO Cells.
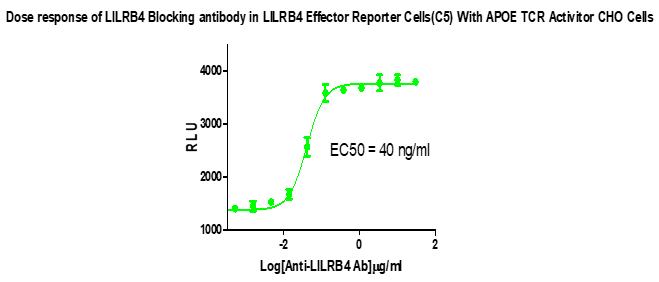
Figure 2. Dose response of LILRB4 Blocking antibody in LILRB4 Effector Reporter Cells(C5) With APOE TCR Activitor CHO Cells.
LILRB1 Effector Reporter Cell RQP74187
B2M-associated HLA-G/aAPC Cell RQP74189

Figure 3. Dose Response of LILRB1 Blocking Antibody in LILRB1 Effector Reporter Cells (C13)With B2M-associated HLA-G/aAPC Cells (C15).
LILRB2 Effector Reporter Cell RQP74188
B2M-associated HLA-G/aAPC Cell RQP74189
B2M-free HLA-G/aAPC Cell RQP74190

Figure 4.Dose Response of LILRB2 Blocking Antibody in LILRB2 Effector Reporter Cells (C24) With B2M-associated HLA-G/aAPC Cells(C15).
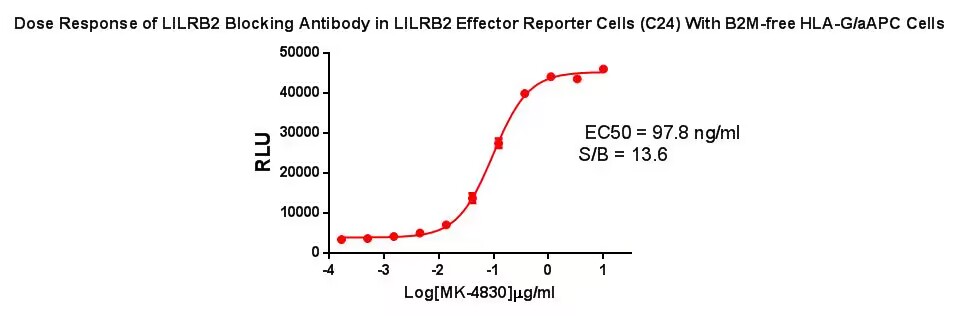
Figure 5. Dose Response of LILRB2 Blocking Antibody in LILRB2 Effector Reporter Cells (C24) With B2M-free HLA-G/aAPC Cells.

