Potential targets for the treatment of pain—— NGF
Background
Chronic pain has always seriously plagued people's physical and mental health, bringing huge public health impact and economic burden to the society. Although the mortality rate of cardiovascular disease, cancer and diabetes is high, pain and many chronic pain-related diseases even if they do not It will be life-threatening immediately, but it is also one of the main sources of human pain, so chronic pain is also called non-fatal cancer. At present, the drugs for the treatment of chronic pain are mainly non-steroids and opioids, but their efficacy is very limited, less than 30% of chronic pain patients can benefit from them, and it is accompanied by many toxic and side effects. Therefore, there is a need to develop new therapeutic drugs imminent.
Introduction to NGF
Studies have shown that NGF plays a key role in peripheral hyperalgesia and inflammation, participates in the regulation of chronic pain, and is a potential target for chronic pain treatment. NGF was first identified in nociceptive primary neuron/fiber development and was later found to play a role in inflammatory hyperalgesia in adults. Since its discovery, more and more studies have proved that NGF is involved in the occurrence of musculoskeletal pain, arthritis pain and cancer pain. NGF levels were found to be significantly elevated in pain states, including inflammatory pain and tumor-related pain, and NGF mRNA was also found to be significantly increased in the skin and muscle of some surgical incisions. Selective antagonists of NGF have been shown to be highly effective in animal models of various acute and chronic pain states without significant side effects.
Nerve growth factor (NGF) is a nerve growth factor stimulated and released by peripheral tissue injury, and its expression level is usually increased in chronic neurological diseases. NGF binds to its receptor TrkA, activates the phosphorylation of TrkA intracellular domain kinase and downstream signaling pathways, and leads to the production of neurotransmitters and ectopic discharge, resulting in the occurrence of pain. Based on the above mechanism, NGF is a potential target for pain therapy.
Studies have shown that anti-NGF antibodies can interfere with the nervous system by blocking the binding of NGF to its receptors, thereby exerting an analgesic effect. Therefore, many domestic and foreign pharmaceutical companies are developing antibody drugs against NGF for pain treatment. Tanezumab, jointly developed by Pfizer/Eli Lilly, was the first NGF antibody drug to enter the clinic, but its road to the market was blocked several times due to its safety issues. The problem encountered challenges, and the clinical trial was stopped by the FDA. Although safety issues have become the main bottleneck in the development of this target drug, as a promising next-generation analgesic drug target in the field of pain, it still cannot stop the continuous stream of latecomers. Absolute research and development enthusiasm. It is worth mentioning that last year, the U.S. FDA approved Solensia (Frunevetmab injection), an NGF monoclonal antibody drug, to control pain associated with osteoarthritis in cats. Although it is an animal drug, it is also a success of NGF monoclonal antibody. The subsequent research and development of NGF antibody drugs for human pain treatment also brought certain confidence. At present, the NGF antibodies of many domestic companies have obtained clinical approval from NMPA, including DS002 from Dashi Pharmaceutical, SMR7694 from Unnamed Biotech, EP-9001A from Urobiology, a subsidiary of Yuandong Biology, and Akeso Biotech. AK115, Technomicrobio's TNM009, etc.
Aiming at this important target for the treatment of pain, we have developed a cell screening model for this target, which can be used to measure the functional activity and evaluate the efficacy of NGF antibody at the cellular level. In addition, this model can also be used for NGF receptor TRKA small Screening of molecular inhibitors, product information and related data are as follows:
NGF/hTRKA Effector Reporter Cell RQP74174
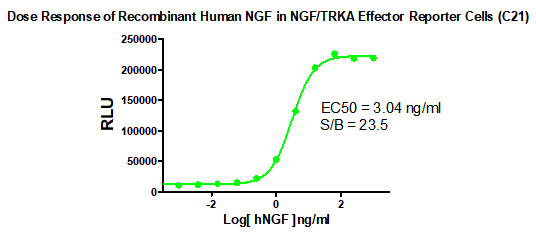
Figure 1. Dose Response of Recombinant Human NGF in NGF/TRKA Effector Reporter Cells (C21).
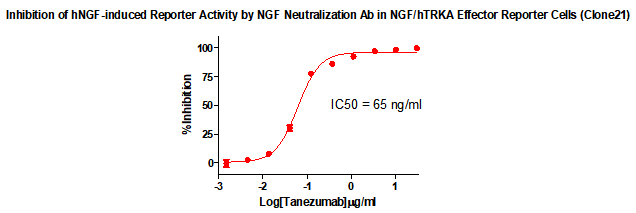
Figure 2. Inhibition of hNGF-induced Reporter Activity by NGF Neutralization Ab in NGF/hTRKA Effector Reporter Cells (Clone21) .
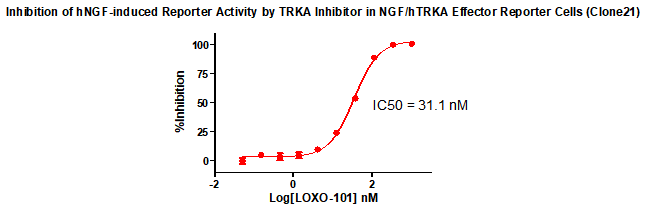
Figure 3. Inhibition of hNGF-induced Reporter Activity by TRKA Inhibitor in NGF/hTRKA Effector Reporter Cells (Clone21) .

