Double-sided PD1, Blocker has become king, have you gotten on the agonist track yet?
PD-1 is one of the most popular targets in recent years. The products on the market are all new drugs. The research and development track can be described as the "king of involution". However, there are still many well-known pharmaceutical companies entering the game one after another, and the price war has intensified. situation. The global antibody drug development track of PD-1 inhibitors has undoubtedly entered the "Red Sea", and a dark battle may be raging.
According to Yao Rongyun statistics, as of November 2022, a total of 15 PD-1 drugs have been approved for marketing worldwide, all of which are PD-1 inhibitors, including Innovent’s sintilimab, Hengrui’s Camrelizumab from Pharmaceuticals, Toripalimab from Junshi Biotech, Cepalimab from Yuheng Pharmaceutical, Tislelizumab from BeiGene, and Piamp from Zhongshan Kangfang Biotech Limumab, cardonilimab and putalimab, pembrolizumab from Merck, nivolumab from Ono Pharmaceuticals, slulimab from Henlius, cemiplimab from Regeneron, Bristol-Myers Squibb's BMS-986213, Biocad Ltd's prolgolimab, and AnaptysBio Inc's dostarlimab.
In the ranking of PD-1 inhibitor sales in 2021, the top 6 drugs accounted for 99.61% of sales, namely sintilimab (30.97%), camrelizumab (28.25%), pembrolizumab Monoclonal antibody (14.52%), tislelizumab (12.31%), nivolumab (7.35%), toripalimab (6.48%). That is to say, starting from the seventh PD-1 inhibitor, it is almost impossible to drink soup.
There is no doubt that PD-1/PD-L1 is a good target that is once in a century. For the involution of PD-1/PD-L1 inhibitors, we need to find a way to break the situation, which can be simply divided into the following categories:
1. Continue to develop drug resistance against PD-1/PD-L1 inhibitors;
2. Develop small molecule drugs targeting PD-1/PD-L1, after all, small molecules have better tissue permeability;
3. Develop anti-PD-1/PD-L1 double antibodies, or even third anti-antibodies. It is reported that Akeso’s PD-1/CTLA-4 double antibody new drug Kaitanil will have a sales volume of 546 million in the first half of 2022; 4. In the face of autoimmunity Disease market, developing agonist drugs for PD-1. Today we mainly focus on the agonist of PD-1.
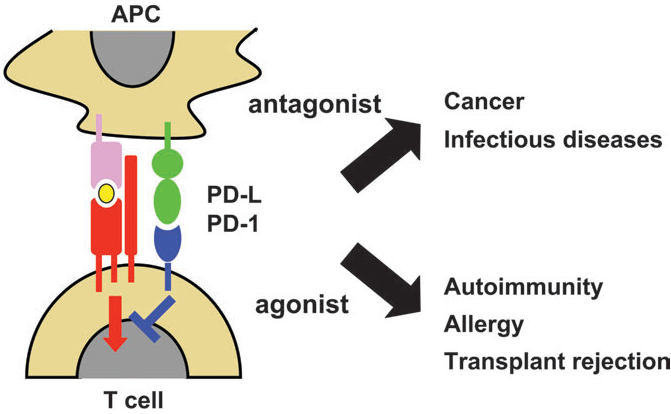
Fig. PD-1/PD-L1的双面性
The so-called autoimmune diseases refer to rheumatoid arthritis, psoriasis, systemic lupus erythematosus and other diseases. Simply put, this type of disease is due to the fact that the immune system is too strong, and it can also kill its own tissues while killing foreign pathogens.
The agonist of PD-1 will inhibit the function and proliferation of immune system guard T cells, preventing the immune system from being too powerful to kill the human body. Contrary to the fire of PD-1 inhibitors, the demand for PD-1 agonists for autoimmune therapy drugs has not been met so far, and it still belongs to the blue ocean market, but the number of entrants has begun to increase.
The first is multinational pharmaceutical giants, such as Eli Lilly, Merck, Bristol-Myers Squibb, and Johnson & Johnson. They have invested a lot of time and money in the research and development of PD-1 agonists, but they have not yet achieved breakthroughs. Among them, the fastest progress is Lilly's PD-1 agonist LY3462817, whose clinical research on patients with rheumatoid arthritis has entered phase II. Although the PD-1 agonist CC-90006 of Celgene, a subsidiary of Bristol-Myers Squibb, completed the phase I clinical study as early as 2019, the project has not progressed since then. Merck’s PD-1 agonist came through mergers and acquisitions. In February last year, Merck acquired Pandion for US$1.85 billion and obtained two PD-1 agonists. One is the systemic PD-2 inhibitor PT627, and the other is the local PD-1 agonist PT001. The former is water-soluble and stable and can be administered systemically, while the latter can be administered locally.
Next to bet on PD-1 agonists are small biotech innovators. ANAPTYSBIO's Rosnilimab, the drug has now started a phase 2 trial for the treatment of moderate to severe alopecia areata, and clinical results are expected in the first half of 2023.
On the domestic front, few pharmaceutical companies have deployed PD-1 agonists, and they are basically still working together to develop PD-1 inhibitors. The main reason is that the research and development difficulty and risk of the latter are much higher than those of the former. The main reasons are 1. There is not much successful experience to learn from on the road of activating immune checkpoints; 2. There are still many unanswered questions about activating immune checkpoints to treat autoimmune diseases; 3. Side effects caused by potential off-target effects of PD-1 agonists are also worthy of attention.
What we bring to you is a cell-based assay model for detecting PD-1 agonist activity. It is a complete set of solutions, suitable for early drug activity detection and QC release activity detection. The entire assay is carried out around the co-culture model of effector cells PD1/NFAT-Luc/Jurkat and antigen-presenting cells APC, including two kinds of cells, positive drugs, complete medium (including antibiotic), assay medium, and 96-well white microwells Plate, Luc detection kit, Protocol.
The basic principle of this model is: TCR activator from APC cell activates TCR on Jurkat, guides downstream NFAT regulatory factors, turns on the expression of Luc, and PD-1 acts as a brake on T cells. When activated by agonist, it will inhibit NFAT -The expression of Luc in the Luc pathway, at the same time, the FCγR on the APC cell can anchor the antibody, form a cross-link, specifically participate in the model, activate PD-1, inhibit the expression of Luc, and finally detect it under the action of the Luc detection kit The corresponding Luc signal forms a complete four-parameter fitting curve, which characterizes the EC50 of the drug and characterizes the window of the assay. This model is a homogeneous and high-throughput cell-based model, which is very suitable for early multi-sample activity detection. At the same time, the model has a large window and good stability, and is also suitable for experiments in the QC release phase.
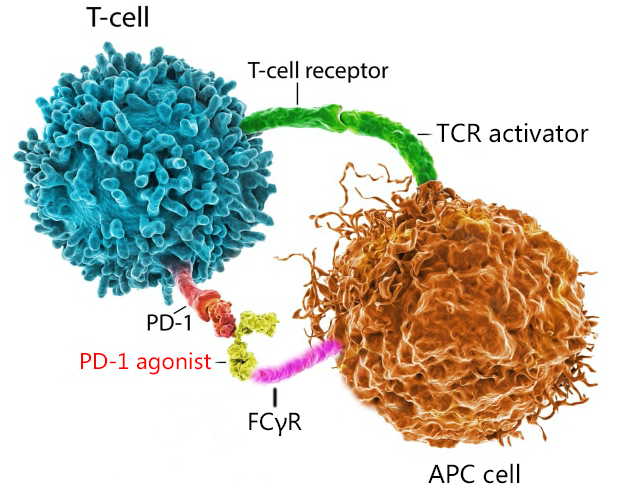
Fig 3. PD-1 agonist assay model principle
Fig 4. Dose Response of PD-1 Agonist Antibody(Peresolimab/LY3462817) in PD-1/NFAT-Luc/Jurkat Cells With FCγR aAPC Cell.

