IL-11 drug cell model
Background
IL-11介绍
IL-11 activates downstream downstream signaling pathways by binding to the IL11RA and gp130 receptor complex on the cell membrane. It activates the following three signaling pathways after binding to its receptor:
1. JAK-STAT3 signaling pathway;
2. RAS-RAF-ERK signaling pathway;
3. PI3K-AKT-mTORC1 signaling pathway.
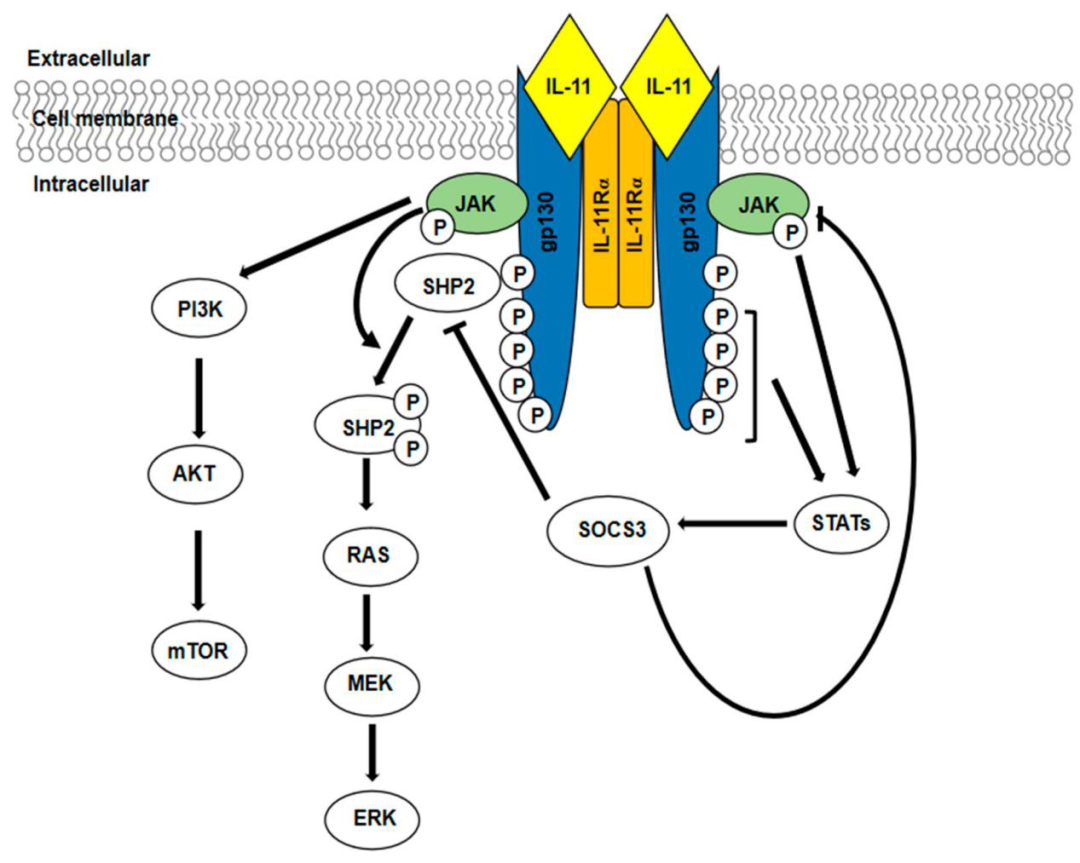
The role of IL-11 is more complex, the main functions include:
1. Stimulate the proliferation, maturation and differentiation of bone marrow hematopoietic stem cells alone or in cooperation with other cytokines;
2. IL-11 stimulates the proliferation of plasma cells and the development of T cells dependent on B cells, and promotes the production of B cell antibodies;
3. Promote the formation and maturation of megakaryocytes, increase the number of peripheral blood platelets;
4. Promote the production and differentiation of red blood cells;
5. Regulate the expression of plasma protein genes in hepatocytes and induce acute phase protein production;
6. Plays an important role in the process of lung inflammation;
7. Inhibit lipoprotein lipase (lipoprotein lipase, LPL) activity and differentiation of adipocytes.
IL-11药研发现状
The currently marketed IL-11 drugs are all recombinant IL-11 proteins, which are used to treat chemotherapy-induced myelosuppression and related anemia, leukopenia and thrombocytopenia. It was first successfully developed by the Genetics Institute in the United States, and it was approved by the FDA in 1997. The trade name is Neumega. It is currently an effective drug for the treatment of thrombocytopenia caused by chemotherapy. A large number of similar drugs have been marketed at home and abroad.
In recent years, more and more studies have found that IL-11 is related to human fibrotic diseases and tumor microenvironment, including nonalcoholic steatohepatitis (NASH), interstitial lung disease (ILD), idiopathic pulmonary Fibrosis (IPF), so the development of IL-11RA antibody drugs has become a new hotspot. A number of pharmaceutical companies have entered the clinical trial stage, among which Lassen Therapeutics' anti-IL-11R monoclonal antibody (LASN01) (currently in clinical phase 1) and Enleofen's IL-11 blocking antibody (currently in the preclinical research stage) ).
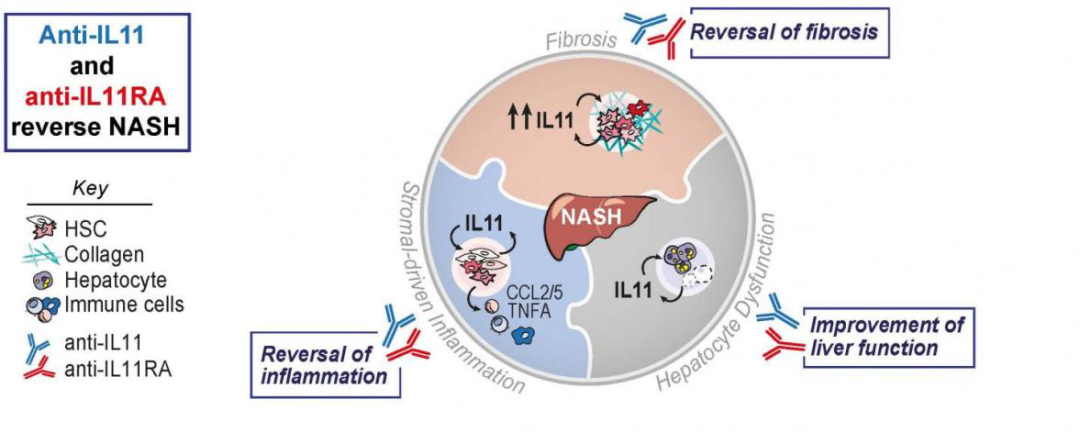
IL-11药物细胞模型
In response to the demand for the development of IL-11 antibody drugs and the quality control release of existing recombinant IL-11 protein drugs, Kebai Biotech has developed the IL11 Effector reporter cell line. Part of the data is shown below:
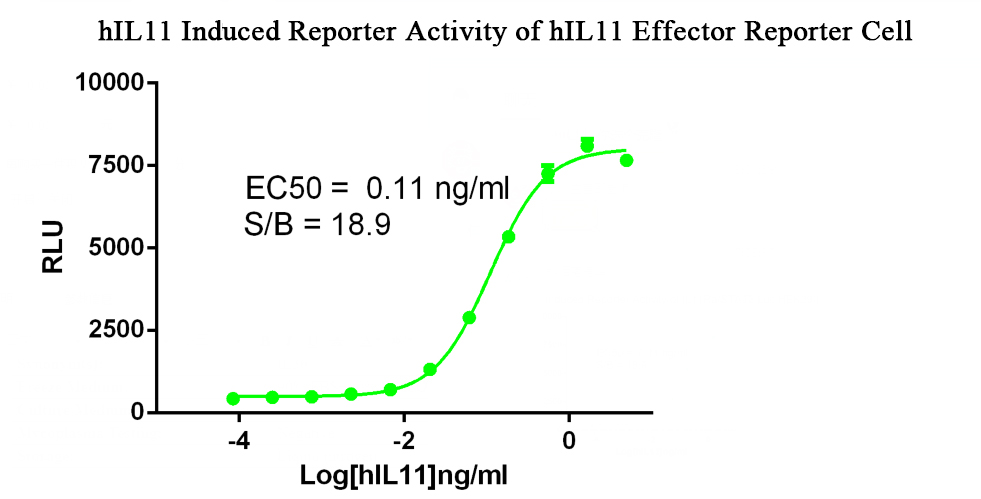
Figure 3. hIL11 Induced Reporter Activity of hIL11 Effector Reporter Cell.

