Tigit & PVRIG dual target cell screening model
Background
The internal immune regulation of the body has a decisive impact on the occurrence and development of cancer. In many solid tumors, the effect T cells have exhausted phenotypes in the micro -environment of the tumor and cannot mediate effective anti -tumor reactions. Such exhaustive T cells often have the characteristics of high expression of co-inhibitory receptors (such as PD-1, CTLA-4, etc.), and antibody therapy that blocks these coexistence receptors and its ligands. Patients show a certain clinical effect, because this is because these common inhibitory experience will relieve the immune suppression of tumor T cells, and lead to an amplification of tumor reactive T cells, restore the immune killing effect of T cells on tumor cells Essence Although the immune examination point inhibitor has brought revolutionary changes to cancer treatment, most patients still have no response to the existing treatment, and many patients who initially responded Drug resistance, therefore, by increasing the understanding of tumor immune response and the discovery of new test targets, it may bring more new treatment options to the majority of cancer patients.
Tigit and PVRIG Introduction
CTLA4 and PD-1 are the earliest developed and applied to tumor immunotherapy targets, while subsequent multiple co-inhibitors of immune checkpoint targets have been successively discovered and used for related tumor immunotherapy development, including a combination of combination Members of protein and class binding protein family: Tigit, PVRIG, and CD96 (Tactile). In addition to the above-mentioned co-inhibiting immune checkpoint receptor, the family also includes a co-stimulating receptor DNAM-1, which can be combined with two ligand PVR (CD155) and PVRL2 (CD112); Tigit, PVRIG and CD96 (Tactile) Together in the combination of appreciation with DNAM-1, it combines its ligands to inhibit its common stimulus signals, thereby generating immunosuppression. Among the above -mentioned inhibitory receptors, TIGIT is the most well -known. It is expressed on T cells and NK cells. Inhibit T cells and NK cell reactions. Dozens of anti-TIGIT monoclonal antibody drugs are currently under development, and some clinical benefits are displayed in clinical experiments when combining anti-PD-L1 drugs. However, the effect of anti-Tigit therapy's single drug has very limited effects. The family's other inhibitory of the receptor PVRIG has high affinity with PVRL2. Through its binding to inhibit the T cell function, the inhibitory effect is not conflict with the inhibitory effect of TIGIT/PVR/PVRL2. The remaining T -cell negative pathway. The in vitro research results also show that Tigit antibodies and PVRIG antibodies can increase T cells amplification or IFN-γ secretion, respectively, and the combination of the two can produce superimposed or synergistic effects to further improve the T cell function.
Research and development status
These pre -clinical studies have greatly supported the Tigit/PVRIG dual -blocking tumor immunotherapy development strategy to a large extent. As one of the main discoverers of the Israeli biotechnology company, COMPUGEN, the PVRIG antibody drug COM701 has been developed. Pre-clinical studies have shown that the COM701 combined with PD1 or tigit inhibitors can significantly enhance the T cell function. At present, the company has carried out clinical experiments for the treatment of solid tumors for the treatment of solid tumors. In China, Jun Shi Bio-targeted PVRIG antibody drugs JS009 also obtained clinical trial approval in August. The use of Ripley Mipide can further promote the activation of T cells and improve the effects of clinical treatment. Therefore, it is planned to actively explore various solutions for joint medications in the follow -up, in order to play a synergistic anti -tumor role in independent research and development products.
With the development and application of special anti-anti-technologies in recent years, several TIGIT/PVRIG dual-anti-drug drugs are also accelerating development. At present, there are three dual anti-drugs for TIGIT/PVRIG. The clinical application was declared on September 17, 2021. It is currently in the clinical stage of Phase 1. It is the clinical Tigit/PVRIG dual anti -drug; the PM1009 of the Pribis creatures was declared clinical on July 11, 2022. Phase; last month, Xianyin Pharmaceutical submitted clinical applications for its independently developed TIGIT/PVRIG dual anti -drug SIM0348.
Faced with the current development status of such as Tigit & PVRIG antibody drugs, especially the development needs of two -specific antibody drugs, we has developed Tigit & PVRIG dual -target cell screening models, which can be used by cell horizontal functional and evaluation of Tigit & PVRIG combination drugs and the combination of Tigit & PVRIG. The efficacy of dual -characteristic antibodies, product information and related data are as follows:
TIGIT/PVRIG Dual Effector Reporter Cell CELL RQP74167
CD112/CD155 Dual AAPC CELL RQP74168
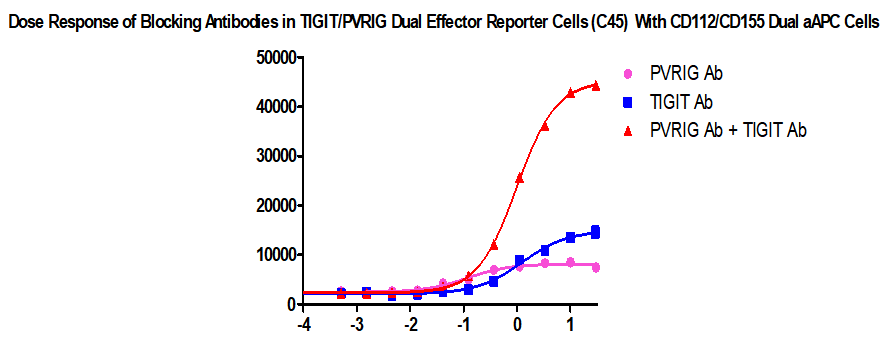
FIGURE 1. Dose Response of Blocking Antibodies in Tigit/PVRIG Dual Effector Reporter Cells (C45) With CD112/CD155 Dual Aapc Cells.

