[Product Promotion] RANKL Antibody Screening Model
Introduction to RANKL/RANK signaling pathway and disease correlation
Nuclear factor receptor-κB activator (RANK/TRANCE receptor (RANK/TRANCE receptor/TNFRSF11A) is a member of the tumor necrosis factor receptor (TNFR) family. The binding of its ligand (RANKL) to the receptor can be increased by Bone turnover regulates the formation, activation and survival of osteoclasts in the process of bone modeling and remodeling, and participates in the pathological process of several related diseases.
The osteoclastogenesis signaling pathway is activated by osteoblasts that produce RANKL. RANKL binds and activates the RANK receptor of the osteoclast precursor. Then the adaptor protein TRAF6 is recruited to the RANK receptor and activates NF-κB, which induces it to enter the nucleus. Promote the increase of c-FOS and NFATc1 expression, thereby enhancing the transcription of genes related to osteoclastogenesis. Osteoprotegerin (OPG) can bind to and inhibit the signal pathway induced by RANKL. In cells with too much RANKL or insufficient OPG, the up-regulation of RANKL/RANK signals can lead to excessive osteoclast formation and bone resorption, causing pathological changes. Bone loss and destruction.
In addition, RANKL also plays a role in certain cancers. In osteosarcoma, in addition to cancerous bone destruction, RANKL is also involved in tumor occurrence and metastasis; studies have also shown that RANKL inhibition can significantly delay carcinogenesis in mouse models of breast cancer And hormones induce the formation of breast tumors.
Development and current status of RANKL inhibitor drugs
According to the mechanism of the RANKL/RANK signaling pathway involved in related diseases, RANKL neutralizing antibodies have been widely developed as inhibitors of the RANKL/RANK signaling pathway, and are used clinically to include osteoporosis, tumors and related complications And other treatments for different indications. As the first approved monoclonal antibody against RANKL, Denosumab has been approved for use in multiple clinical indications, and has resulted in corresponding different trade names (as shown in the table below). Show).
With the approval of several indications of desulumab in China, it has also triggered the follow-up development of more than ten domestic companies for different indications-related varieties. Among them, mainly biosimilar drugs are mainly used, and a few are optimized by biobetter. product. The following table is a summary of the progress of some RANKL antibody products of domestic companies.
For the research and development of RANKL inhibitor antibody drugs, we have developed related cell screening models, which can be used for functional cell-level screening and live measurement.
RANK Effector Reporter cell RQP74125
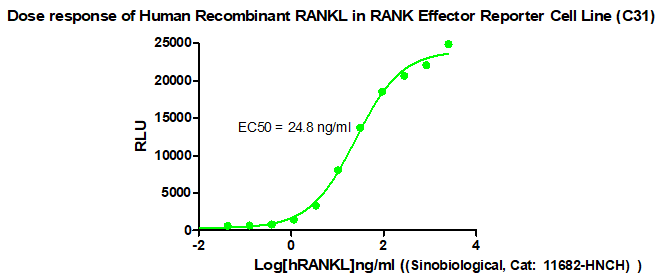
Figure 1. Dose response of Human Recombinant RANKL in RANK Effector Reporter Cell Line (C31).
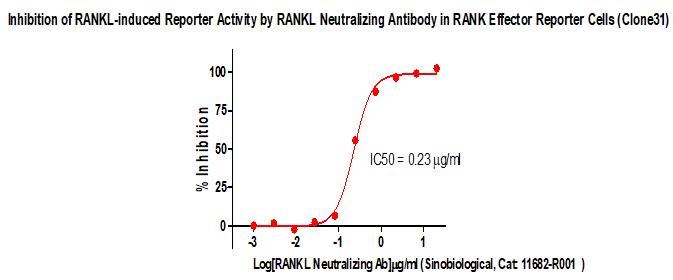
Figure 2. lnhibition of RANKL-induced Reporter Activity by RANKL Neutralizing Antibody in RANK Effector Reporter Cells (Clone31).

